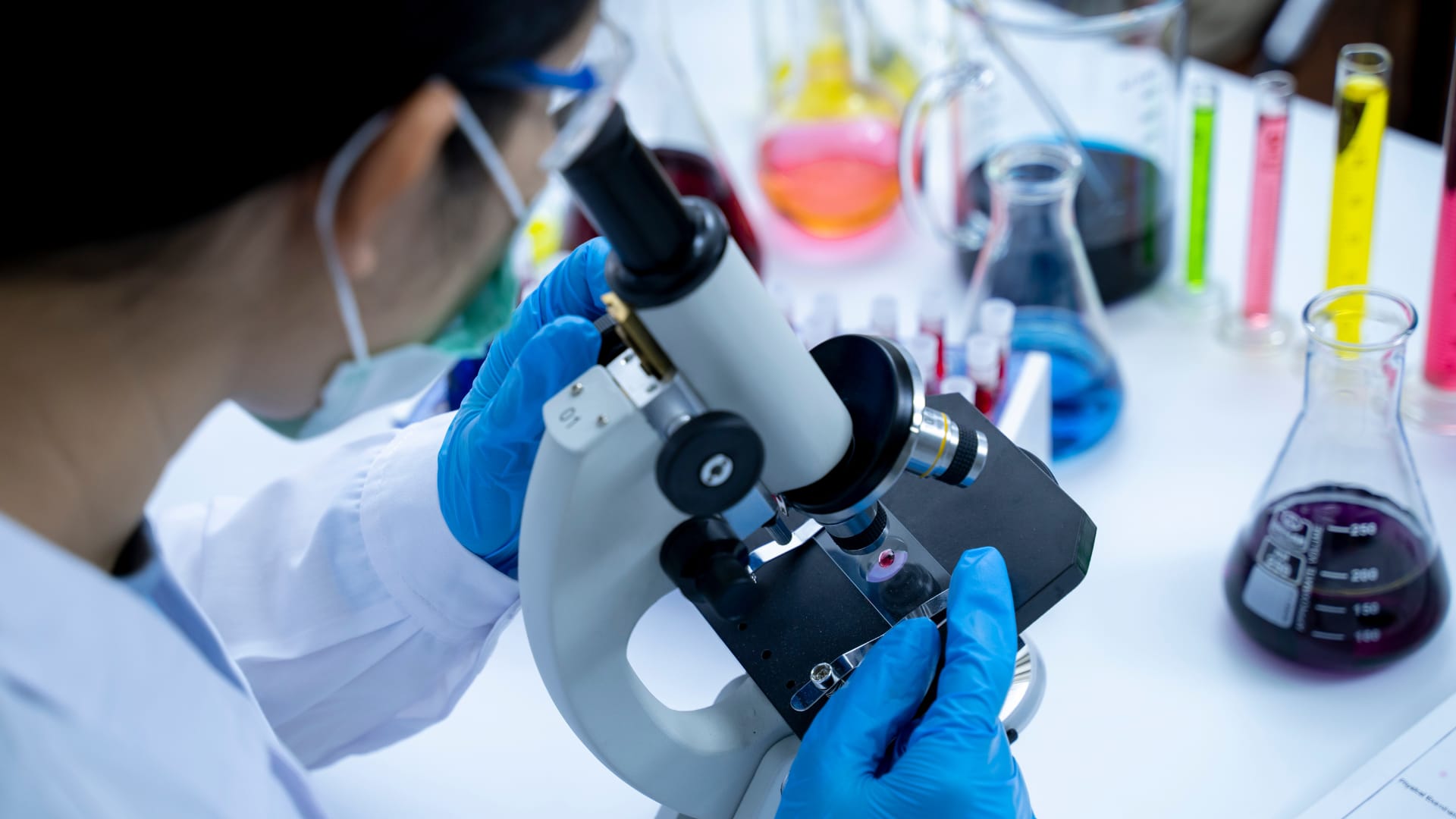
CAR T cell-therapy, hope and healing: Dean’s story

In August 2024, Dean Lookingbill and his wife Mary had the unique experience of touring a facility in Seattle that manufactures T cells for cancer treatment. The Lookingbills were in awe as they watched a technician’s gloved hands work with precision.
Three years prior, Dean had undergone CAR T-cell therapy treatment at Providence Cancer Institute of Oregon for lymphoma. After removing the T cells from his body, they were sent to the Seattle facility to be engineered for his treatment. Dean wondered if his T cells had been handled by the meticulous technician in the lab.
Multiple cancer diagnoses
In 2018, at age 66, Dean was diagnosed with large B cell non-Hodgkin's lymphoma, and he received six chemotherapy treatments over five months. When a PET scan showed his cancer was gone, Dean and Mary exhaled. They threw themselves back into their busy lives in Vancouver, WA., filling their days with family, putting extra time in with their grandkids.
Two years later, just when the routines of family life felt steady again, Dean learned he had prostate cancer. Over the next several months he went through routine scans to monitor the cancer. Then a mass was discovered during one of the scans – the lymphoma had returned. Although Dean’s resolve was tested, he was determined to face the next challenge head-on.
Dean was referred for stem cell therapy to Providence Cancer Institute medical oncologist, John Godwin, M.D., MS, a specialist and researcher in blood cancers.
“Dr. Godwin told me I could receive stem cell treatment,” says Dean. “But first I had to go through an intense chemotherapy treatment.”
Stem cell therapy involves replacing the damaged cells in a patient’s bone marrow with healthy cells from a donor. It’s a complex process that starts with intense chemotherapy to clear out the cancer cells, preparing the body for new, healthy cells to grow.
Dean received two rounds of chemotherapy. He experienced some challenging side effects but tolerated it fairly well. He and Mary were feeling confident when they met with Dr. Godwin to review scans and discuss next steps. But the news Dr. Godwin shared with them wasn’t what they had hoped. “He told us it didn’t work well,” says Dean. “Then in his next breath, he looked at me and said, ‘But don't lose hope.’”
Dr. Godwin told them about CAR T-cell therapy, a relatively new treatment available to patients with certain types of cancer. Dean would be the third patient at Providence Cancer Institute to receive CAR T-cell therapy.
What is CAR T-cell therapy?
 CAR T-cell therapy involves extracting a cancer patient’s white blood cells (T cells) and reprogramming them with an artificial receptor called a chimeric antigen receptor (CAR). The modified cells are multiplied in a lab and then returned to the patient’s body where they latch on to and kill malignant cells.
CAR T-cell therapy involves extracting a cancer patient’s white blood cells (T cells) and reprogramming them with an artificial receptor called a chimeric antigen receptor (CAR). The modified cells are multiplied in a lab and then returned to the patient’s body where they latch on to and kill malignant cells.
In 2018, Providence Cancer Institute became the first of 13 U.S. centers to join a CAR T-cell therapy trial for patients with relapsed or treatment-resistant B-cell non-Hodgkin lymphoma—the type of cancer Dean had.
Dr. Godwin led the phase II OUTREACH trial, testing the safety and effectiveness of a CAR T-cell therapy called liso-cel, or its brand name Breyanzi, in outpatient and inpatient settings. The study results were published in multiple peer review journals and presented at oncology and hematology conferences.
Supportive, consistent care
At Providence Cancer Institute, the first step in CAR T-cell therapy is an information session. Dean and his family met with Ian Malkasian, RN, clinical program manager, and Krista Nelson, LCSW, oncology social worker. For the next 90 minutes, Ian and Krista did more than just explain the treatment. “There are a lot more moving parts with T-cell therapies and cellular based therapies,” says Ian.
Dean’s family was guided through each stage of the process, from the initial tests and blood draw to the infusion, plus what to expect in the 30 days that follow the infusion. They gave a brief history of the clinical trial that led to the treatment and its potential side effects. Ian and Krista ensured that Dean and his family felt informed and prepared.
While Krista’s check-ins brought emotional support, Ian made sure every appointment, test and detail was handled. He served as the liaison with the versatile care team, connected Dean and Mary with resources and many other supportive tasks. “There is a significant logistical component to this, but also an emotional and psychosocial component because patients get nervous,” says Ian. Ultimately, his goal was to reassure Dean and Mary and support them at every point of the journey – and they felt it throughout the process.
Deans says he and Mary trusted Ian, Krista and the care team from the moment he started his care at Providence Cancer Institute. “I can’t tell you how important that part of the caregiving process is for patients.”
Waiting for treatment results
In July 2021, Dean’s T cells were isolated and genetically reengineered in the Seattle lab. Then the newly fortified army of T cells was returned to his body through an infusion. For the next month, Dean and Mary stayed at a hotel in the Pearl District near downtown Portland. Providence Cancer Institute requires CAR T-cell patients to remain within a 30-minute radius of the clinic for 30 days. This is to ensure patients can get care within minutes if they experience severe side effects. During that period patients are closely monitored, and they must have a caregiver with them or nearby around-the-clock. The Lookingbill’s hotel expenses, including daily per diem, were paid for by Bristol-Meyer Squibb, the manufacturer of Breyanzi, the medication used in the treatment.
Luckily, Dean did remarkably well, and he and Mary made the most of their stay. Family visits and sitting poolside on hot summer days made their time away from home bearable. They explored downtown Portland and ate at restaurants in the area.
Then it was time for a PET scan. After the scan, Dean and Mary sat down in Dr. Godwin’s office to look at the results. “I thought, ‘this is it, the last inning,’” says Dean. He prepared himself, feeling a mix of apprehension and hope.
Dr. Godwin entered the office. “He looked at me and said, ‘It worked. It’s clear,’” says Dean “I jumped up and gave him a hug.”
Faster turnaround, more accessibility
Dr. Godwin retired in 2022, and since then Dean has been under the care of Natasha Edwin, M.D., hematology oncologist at Providence Cancer Center Oncology and Hematology Care Clinic, Westside Portland.
Dr. Edwin says CAR T-cell therapy has revolutionized the course of cancer care for diffuse large B cell lymphoma, and also leukemia and myeloma. Although myeloma is considered an incurable type of blood cancer, CAR T-cell therapy studies have shown patients are living longer without the cancer returning.
Better patient outcomes have catapulted CAR T-cell therapy from a fifth-line treatment for some blood cancers – meaning it is given only after four rounds of chemotherapy, or a combination of therapies have failed – to a second- or third-line treatment.
Although CAR T-cell therapy is rapidly gaining momentum as a promising treatment for various types of cancer, its effectiveness and accessibility still need improvement. One of the major challenges is the complex and time-consuming process of manufacturing the T cells. Currently, it takes about one month. To address this, researchers are developing faster and more efficient methods and approaches that could make CAR T-cell therapy more widely available to patients.
In fact, Dr. Edwin currently is working on two clinical trials at Providence Cancer Institute for treating myeloma and lymphoma that rely on cells that don’t have to be manufactured. “They’re ‘off-the shelf’ so we can get them to our patients much faster,” explains Dr. Edwin.
Living cancer-free
Today, Dean is clear of lymphoma although he gets a CT scan and blood draw twice a year. "He is technically cancer-free, but we are watching for relapses,” says Dr. Edwin. She explains that the risk of cancer returning is highest in the first couple years after treatment, then it declines.
“Once we hit the five-year mark, we usually tell patients that their risk of relapse from the diffuse large B cell lymphoma is really negligible to the point that we would consider discharging them from regular cancer surveillance,” she says.
In spring 2024, Dean finished his last round of hormonal treatment for prostate cancer. He says life has taken on a deeper meaning – he embraces every moment with Mary and his family and has a deeper sense of hope. Time spent with the grandkids helps him stay focused on the present. “They transport me out of the place of worry,” says Dean.
Providence Cancer Institute: A center of excellence
 In 2024, Providence Cancer Institute was recognized by Becker’s Hospital Review as one of the Top 100 hospitals and health systems for excellence in oncology. This honor reflects our commitment to advancing cancer care through innovation, collaboration and compassion.
In 2024, Providence Cancer Institute was recognized by Becker’s Hospital Review as one of the Top 100 hospitals and health systems for excellence in oncology. This honor reflects our commitment to advancing cancer care through innovation, collaboration and compassion.
Our researchers develop novel immunotherapies, enhance the basic understanding of the biology of cancer and translate those findings into effective new treatments for patients. The majority of our trailblazing research is supported by philanthropy. Your gifts advance the fight against cancer and give hope to cancer patients like Dean and his family.
Discover more about the leading-edge research and care at Providence Cancer Institute.
Related news
Leadership change at Providence Cancer Institute, #FINISHCANCER goal remains
Harnessing CAR T-cell therapy: A new study for indolent B-cell non-Hodgkin lymphoma